What is everything you know about Niobium?
Niobium


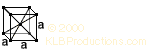



The metal was first prepared in 1864 by Blomstrand, who reduced the chloride by heating it in a hydrogen atmosphere. The name niobium was adopted by the International Union of Pure and Applied Chemicstry (IUPAC) in 1950 after 100 years of controversy. Many leading chemical societies and government organizations refer to it by this name.
Most metallurgists, leading metal societies, and all but one of the leading U.S. Commercial producers, however, still refer to the metal as "Columbium. " Sources The element is found in niobite (or columbite), niobite-tantalite, parochlore, and euxenite. Large deposits of niobium have been found associated with carbonatites (carbon-silicate rocks), as a constituent of parochlore.
Extensive ore reserves are found in Canada, Brazil, Nigeria, Zaire, and in Russia.

Uses Niobium is used in arc-welding rods for stabilized grades of stainless steel. Thousands of pounds of niobium have been used in advanced air frame systems such as were used in the Gemini space program. The element has superconductive properties; superconductive magnets have been made with Nb-Zr wire, which retains its superconductivity in strong magnetic fields.
This type of application offers hope of direct large-scale generation of electric power. Niobium is also commonly used for jewelry.

The metal can be isolated from tantalum, and prepared in several ways. Cost Niobium metal (99.5% pure) is priced at about $75/lb. Picture: The crystal structure of niobium, body-centered cubic

However, plant growing near niobium deposits can accumulate the metal to levels above 1 ppm. Niobium was mined chifely as columbite, and is formerly known as colombium (Cb). Another mined metal is pyrochlore and this is now the most important.
The main mining areas are Brazil, which produce more than 85% on the world's niobium, Zaire, Russia, Nigeria and Canada. World production is around 25.000 tonnes per year. The amount of unmined reserves is not known, but there are extensive deposits of pytochlore.
Health effects of niobium Niobium and its compounds may be toxic (niobium dust causes eye and skin irritation) , but there are no reports of human being poisoned by it. Apart from measuring its concentration, no research on niobium in humans has been undertaken. Niobium, when inhaled, is retained mainly in the lungs, and secondarily in

In laboratory animals, inhalation of niobium nitride and/or pentoxide leads to scarring of the lungs at exposure levels of 40 mg/m3. Environmental effects of niobium No negative environmental effects have been reported.

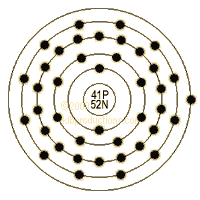
Niobium is element number 41 in the periodic table. It is a transition element (one with more electrons in the next-outermost shell than the preceding noble gas), and is always found with tantalum; hence the name (Niobe was the daughter of Tantalus in Greek mythology). It was once called columbium, and has many industrial uses.
Facts about the Definition of the Element Niobium The Element Niobium is defined as... A silvery, soft, ductile metallic element that occurs chiefly in columbite-tantalite and is used in steel alloys, arc welding, and superconductivity research. This element is still widely referred to by its original name - Columbium. Interesting Facts about the Origin and Meaning of the element name Niobium What are the origins of the word Niobium?
Name Origin - Columbium was the name originally given to this element by Hatchet but IUPAC officially adopted "niobium" as the name originally given by Heinrich Rose in 1846. The word Niobium originates from Niobe, daughter of mythical Greek king Tantalus. Facts about the Classification of the Element Niobium Niobium is classified as a "Transition Metal" which are located in Groups 3 - 12 of the Periodic Table.An Element classified as a Transition Metals is ductile, malleable, and able to conduct electricity and heat.
Brief Facts about the Discovery and story of the Element Niobium Niobium was discovered by Charles Hatchet in 1801 in the columbite ore that was sent to England in the 1750s by John Winthrop, the first governor of Connecticut. Hatchet therefore gave it the name Columbium. Heinrich Rose and Jean Charles Galissard de Marignac rediscovered the element in 1846.
Christian Blomstrand was the first to prepare the metal in 1864. Occurrence of the element Niobium in the Atmosphere Obtained from columbite Common Uses of Niobium Tantalum capacitor Steel alloys Tantalum plating Hot metal spraying Arc welding Super-conductivity research The Properties of the Element Niobium Name of Element : Niobium Symbol of Element : Nb Atomic Number of Niobium : 41 Atomic Mass: 92.90638 amu Melting Point: 2468.0 °C - 2741.15 °K Boiling Point: 4927.0 °C - 5200.15 °K Number of Protons/Electrons in Niobium : 41 Number of Neutrons in Niobium : 52 Crystal Structure: Cubic Density @ 293 K: 8.57 g/cm3 Color of Niobium : silvery white The element Niobium and the Periodic Table Find out more facts about Niobium on the Periodic Table which arranges every chemical element according to its atomic number, as based on the periodic law, so that chemical elements with similar properties are in the same column. Our Periodic Table is simple to use - just click on the symbol for Niobium for additional facts and info and for an instant comparison of the Atomic Weight, Melting Point, Boiling Point and Mass - G/cc of Niobium with any other element.
An invaluable source for more interesting facts and information about the Niobium element and as a Chemistry reference guide.
Niobium has similar physical and chemical properties to another element, tantalum, and the two are therefore difficult to distinguish. The English chemist Charles Hatchett reported a new element similar to tantalum in 1801, and named it columbium. In 1809, the English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical.
The German chemist Heinrich Rose determined in 1846 that tantalum ores contain a second element, which he named niobium. In 1864 and 1865, a series of scientific findings clarified that niobium and columbium were the same element (as distinguished from tantalum), and for a century both names were used interchangeably. The name of the element was officially adopted as niobium in 1949.
Name Origin - Columbium was the name originally given to this element by Hatchet but IUPAC officially adopted "niobium" as the name originally given by Heinrich Rose in 1846. The word Niobium originates from Niobe, daughter of mythical Greek king Tantalus. Niobium is classified as a "Transition Metal" which are located in Groups 3 - 12 of the Periodic Table.An Element classified as a Transition Metals is ductile, malleable, and able to conduct electricity and heat.
Common Uses of Niobium Tantalum capacitor Steel alloys Tantalum plating Hot metal spraying Arc welding Super-conductivity research.
A silvery, soft, ductile metallic element that occurs chiefly in columbite-tantalite and is used in steel alloys, arc welding, and superconductivity research. This element is still widely referred to by its original name - Columbium. Name Origin - Columbium was the name originally given to this element by Hatchet but IUPAC officially adopted "niobium" as the name originally given by Heinrich Rose in 1846.
The word Niobium originates from Niobe, daughter of mythical Greek king Tantalus. Niobe was the queen of Thebes. She boasted of her fourteen children to the goddess Leto and as a result, Apollo killed her seven sons and Artemis killed her seven daughters.In her grief, Niobe wept and turned to stone.
I cant really gove you an answer,but what I can give you is a way to a solution, that is you have to find the anglde that you relate to or peaks your interest. A good paper is one that people get drawn into because it reaches them ln some way.As for me WW11 to me, I think of the holocaust and the effect it had on the survivors, their families and those who stood by and did nothing until it was too late.
Related Questions
Which is more interesting? Niobium, Aluminium, Silver or Platinum?
Facts about Niobium?
How do you stop a Beagle from wanting to eat everything and tear up everything?
Everything that scientists believe today is completely different from everything scientists believed 200 years?
Nothingness is Everything and Everything is Nothingness?
If I change my password and everything I use it for does my new one take over in everything I used it for?
